Calculators Equations & ReactionsQuestion Uiz 9 Acids And Bases 2 Hours Question 3 CONTENT FEEDBACK Aluminum Hydroxide Reacts With Nitric Acid According To The Balanced Equation Below AICOH), 3 HNO Al(NO,)3 3 H2O A 1236 ML Solution Of AI(OH), Is Titrated With A 0180 M Solution Of HNO, Calculate The Molar Concentration Of AI(OH), If The Endpoint Of The Titration Is Reached After The Addition4 H2O ‡ H2 O2 5 Fe(OH)3 HNO3 ‡ Fe(NO3)3 H2O 19 Use the Activity Series of Metals to determine if the following reactions can occur (yes or no) 1 Ag HCl ‡ 2 Mg AgNO3 ‡ 3 Cu HNO3 ‡ 4 Li Mg(OH)2 ‡ 5 Ca NiSO4 ‡ 6 Pb CuSO4 ‡ 7 Which of the above reactions would be the most explosive?
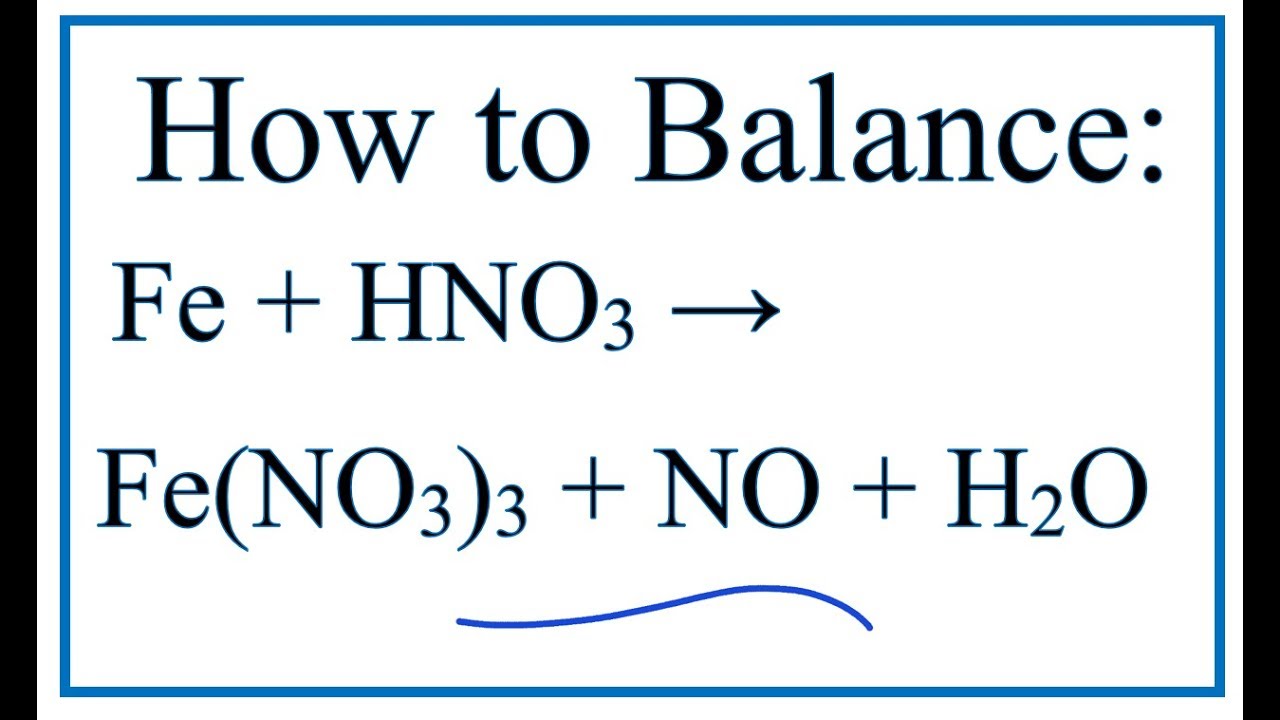
How To Balance Fe Hno3 Fe No3 3 No H2o Iron Nitric Acid Youtube
