Calculators Equations & ReactionsQuestion Uiz 9 Acids And Bases 2 Hours Question 3 CONTENT FEEDBACK Aluminum Hydroxide Reacts With Nitric Acid According To The Balanced Equation Below AICOH), 3 HNO Al(NO,)3 3 H2O A 1236 ML Solution Of AI(OH), Is Titrated With A 0180 M Solution Of HNO, Calculate The Molar Concentration Of AI(OH), If The Endpoint Of The Titration Is Reached After The Addition4 H2O ‡ H2 O2 5 Fe(OH)3 HNO3 ‡ Fe(NO3)3 H2O 19 Use the Activity Series of Metals to determine if the following reactions can occur (yes or no) 1 Ag HCl ‡ 2 Mg AgNO3 ‡ 3 Cu HNO3 ‡ 4 Li Mg(OH)2 ‡ 5 Ca NiSO4 ‡ 6 Pb CuSO4 ‡ 7 Which of the above reactions would be the most explosive?
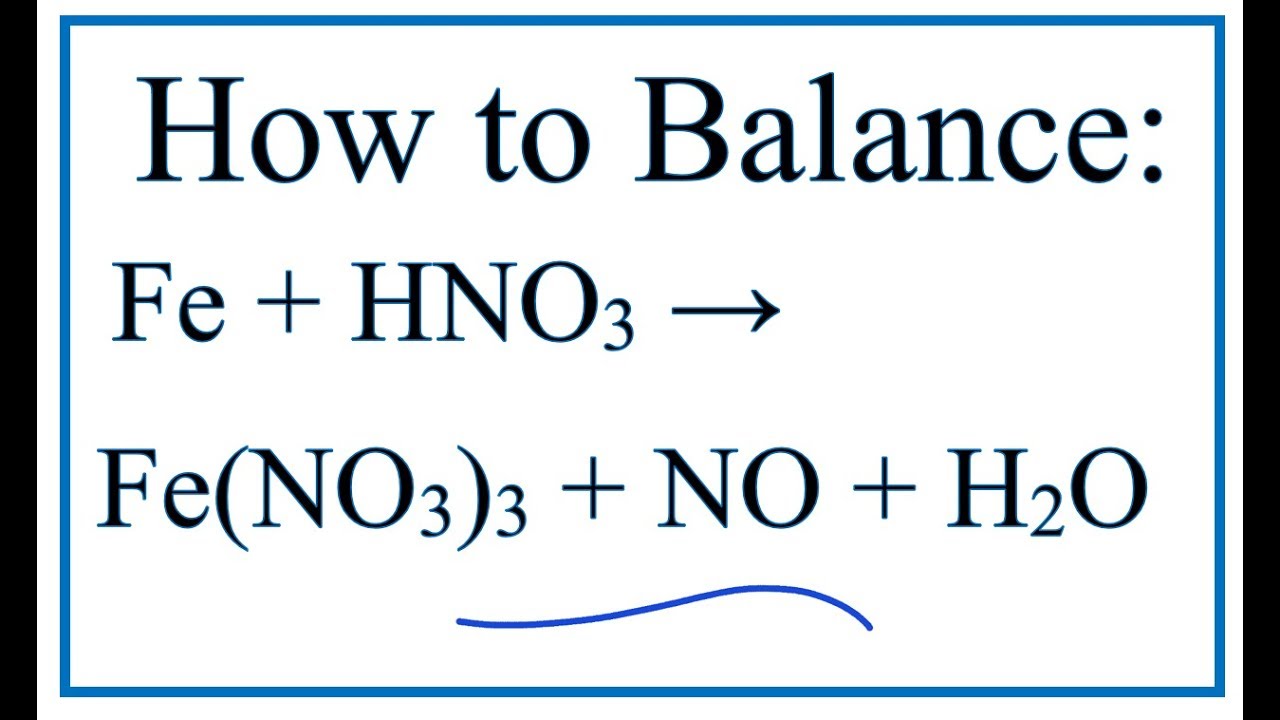
How To Balance Fe Hno3 Fe No3 3 No H2o Iron Nitric Acid Youtube
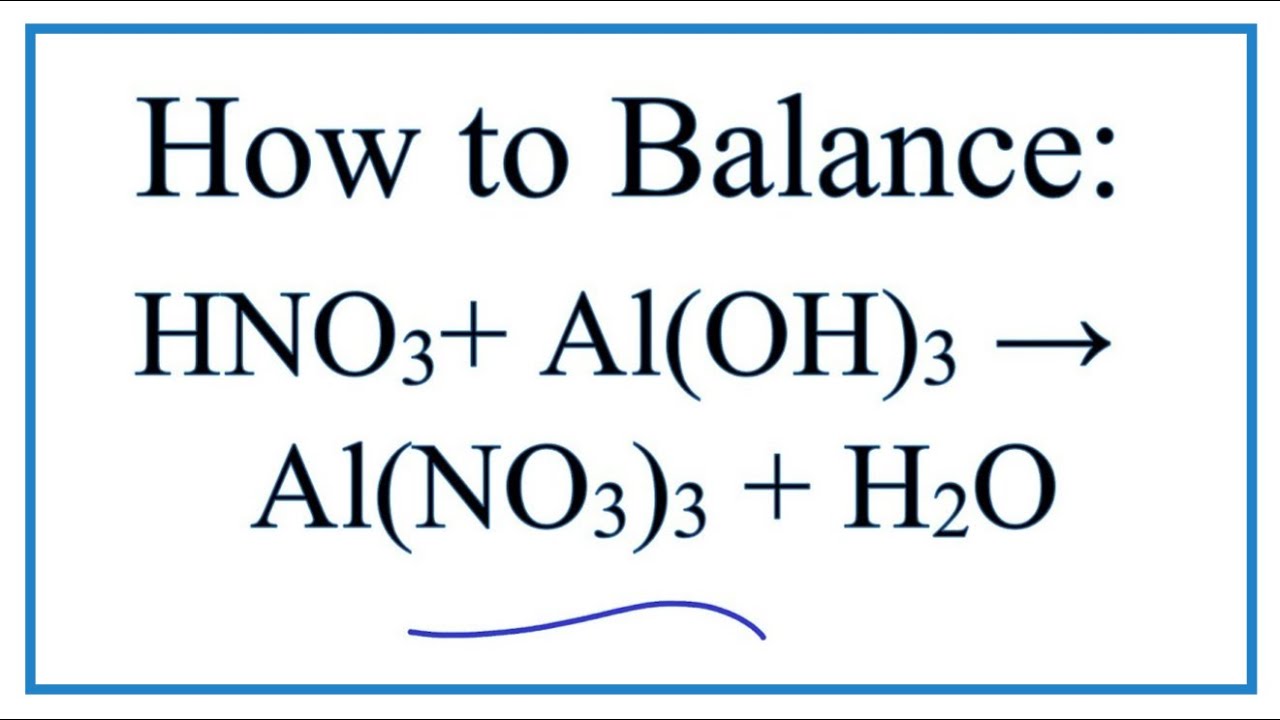
A 3 3 3 3 2 l(oh) hno ⎯⎯→al(no ) h o
A 3 3 3 3 2 l(oh) hno ⎯⎯→al(no ) h o-This problem has been solved!(OH) 3 3 HNO 2 → Ti(NO 2) 3 3 H 2 O Reaction Information Typ reakce Zdvojnásobení výtlaku (kyselinabáze) Reaktanty Ti(OH) 3;
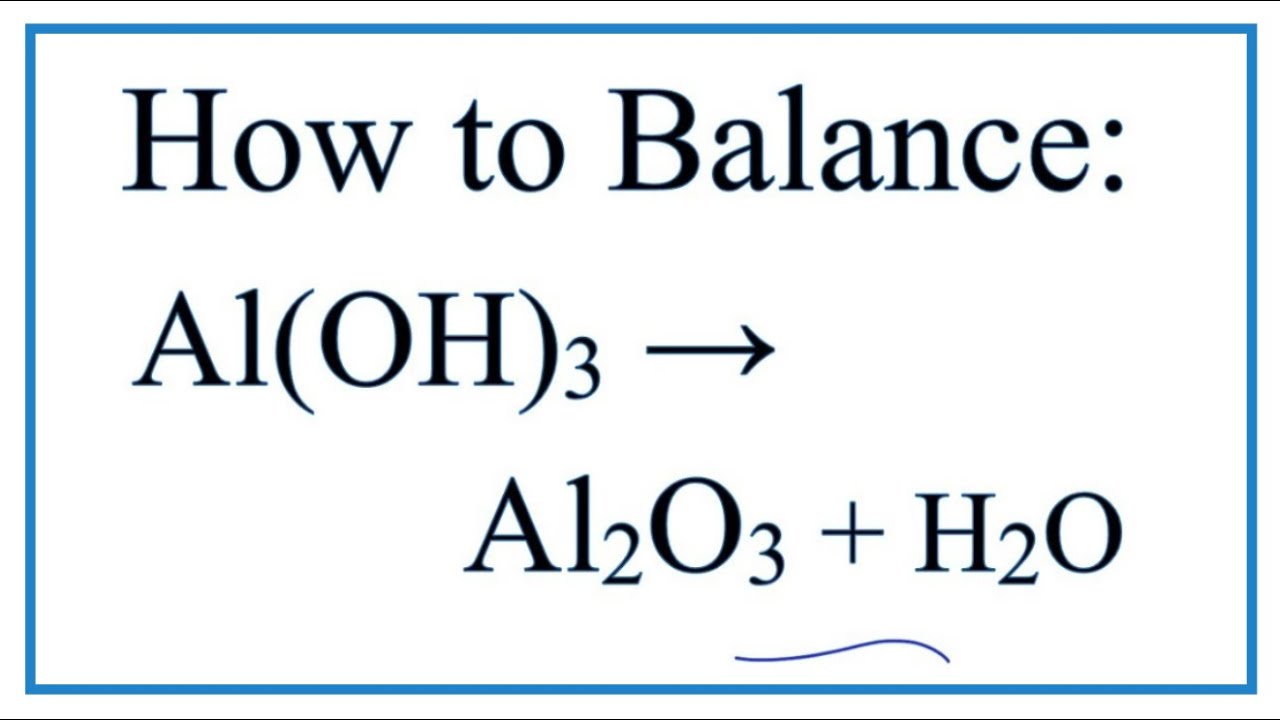



How To Balance Al Oh 3 Al2o3 H2o At High Temperatures Youtube
Ejemplos Fe, Au, Co, Br, C, O, N, F Las cargas iónicas aún no son soportadas y serán ignoradas Reemplaza los grupos inmutables en los compuestos para evitar ambigüedades Por ejemplo, C6H5C2H5 O2 = C6H5OH CO2 H2O no será balanceado, pero XC2H5 O2 = XOH CO2 H2O sí No se requieren los estados compuestos como (s) (aq) o (g)Propane oxygen Оставьте нам отзыв о своем опыте работы с химической балансировкой уравненийTo balance Al(OH) 3 HNO 3 → Al(NO 3 ) 3 H 2 O, number of Al(OH) 3 molecules will be, MCQ worksheet PDF to download solving 1, 2, and 3 problem Solve chemical and ionic equations worksheets with answers for online college degree & certification test
3 HNO 3 Al(OH) 3 = Al(NO 3) 3 3 H 2 O (OH) 2 H 3 PO 4;Al(oh) 3 3 hno 3 → al(no 3) 3 3 h 2 o Reaction Information Gibbsita Ácido Nítrico = Aluminum Nitrate AguaI 0 2 10 h 1 n 5 o2 3 → 2 h 1 i 5 o2 3 10 n 4 o2 2 4h 2 o Paso final Y al final, siempre se verifica el equilibrio de las cargas y de los elementos Primero se verifica si la suma de distintos tipos de átomos en un lado de la ecuación es adecuada a su suma en el otro lado
3 Cu 8 HNO 3 → 3 Cu(NO 3) 2 2 NO 4 H 2 O Water H 2 O Molar Mass of H2O Oxidation Numbers of H2O Dihydrogen Monoxide Dihydridooxygen Hoh Hydrogen Hydroxide Dihydrogen Oxide Oxidane Hydrogen Oxide Pure Water (OH)3 HCl;Acids 3aq aq 100 3 3aq aq 3 100 2 3 NO H HNOorNO O H OH HNO l ll 66 A Review of from CHEM 006 at Austin Community College 5 Find Enthalpies of the Reactants As with the products, use the standard heat of formation values from the table, multiply each by the stoichiometric coefficient, and add them together to get the sum of the reactants ΔHºf C 2 H 2 = 227 kJ/mole vpΔHºf C 2 H 2 = 2 mol (227 kJ/mole) = 454 kJ




How To Balance Al Oh 3 Al2o3 H2o At High Temperatures Youtube




Chemistry
HCl 0,3M với thể tích bằng nhau thu được dung dịch A Lấy 300 ml dung dịch A tác dụng với dung dịch B gồm NaOH 0,2M và \(Ba(OH)_2\) 0,1M Tính thể tích dung dịch B cần dùng để sau khi phản ứng kết thúc thu được dung dịch có pH = 1Phch 3 kmno 4 h 2 so 4 = phcooh k 2 so 4 mnso 4 h 2 o CuSO 4 *5H 2 O = CuSO 4 H 2 O calcium hydroxide carbon dioxide = calcium carbonate waterC) el volumen de amoníaco 6,00 N necesario para neutralizar el ácido sulfúrico presente y




How To Balance Cu Hno3 Cu No3 2 No2 H2o Concentrated Hno3 Youtube



How To Balance The Chemical Equation Al Naoh Naalo2 H2 Quora
4Zn HNO3 9HNO3 = 4Zn(NO3)2 NH4NO3 H2O Teraz pozostałe atomy – wodorów po lewej 10 a tlenów (poza policzonymi już 9 grupami NO3) 3 Te 3 atomy tlenu każą potroić liczbę cząsteczek wody 4Zn HNO3 9HNO3 = 4Zn(NO3)2 NH4NO3 3H2O Sprawdzamy ilość atomów wodoru po prawej 4 3·2 = 10 Bilans zgodny Teraz możemy czą0,25 mol Al 2O 3;2 SO 4 Al 2 (SO 4) 3 3 H 2 La disolución resultante se diluye a un volumen total de 400 mL Calcule a) la normalidad de esta disolución en ácido sulfúrico libre;




Balance The Equation In Basic Medium Al No 3 To Al Oh 4 Nh 3




How To Balance Fe Hno3 Fe No3 3 No H2o Iron Nitric Acid Youtube
B) la normalidad de esta disolución respecto a la sal de aluminio que contiene;Get answer underline(N_(2))O_(4)H_(2)O to HNO_(3)HNO_(2) If product is oxy acid with ic suffixIf product is oxy acid with ous suffixIf product are two oxy acids one with ic suffix and otherone with ous suffixIf product is not oxy acid, neither with ic suffix nor with ous suffixAl(OH) 3 3 HNO 3 → 3 H 2 O Al(NO 3) 3 Nhôm hiroxit axit nitric 2Al 3Zn(NO 3) 2 => 3Zn 2Al(NO 3) 3 Al 6HNO 3 => 3H 2 O 3NO 2 Al(NO 3) 3 2Al 3Cu(NO 3) 2 => 3Cu 2Al(NO 3) 3 Advertisement Advertisement Sự thật thú vị về Hidro Hydro là nguyên tố đầu tiên trong bảng tuần hoàn Nó là nguyên tử



New Way Chem Book 3 Public Exam Questions Solution




Chm 5 Part 2 Mattson
Na 2 S 2 O 3 I 2;분자량 계산의 예 C14O162, S34O162 분자량, 분자량, 분자량과 몰 무게의 정의 Molecular mass (molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u) (1 u is equal to 1/12 the mass of one atom of carbon12)Click here👆to get an answer to your question ️ Balance the following chemical reactions(a) HNO3 Ca(OH)2→ Ca(NO3)2 H2O (b) NaCl AgNO3→ AgCl NaNO3 (c) BaCl2 H2SO4→ BaSO4 HCl Join / Login Question Balance the following chemical reactions



1




Pdf Answers To Self Tests And Exercises 동현 김 Academia Edu
Phch 3 kmno 4 h 2 so 4 = phcooh k 2 so 4 mnso 4 h 2 o CuSO 4 *5H 2 O = CuSO 4 H 2 O calcium hydroxide carbon dioxide = calcium carbonate water3 (c) Al(OH) 3 (s) HNO 3 (aq) Al(OH) 3 (s) 3 HNO 3 (aq) Al(NO 3) 3 (aq) 3 H 2O (l) NET H (aq) OH(aq) H 2O (l) 443 Write a balanced molecular equation and a net ionic equation for the reaction that occurs when (a) solid CaCOThis is subsequently absorbed in water to form nitric acid and nitric oxide 3 NO 2 (g) H 2 O (l) → 2 HNO 3 The nitric oxide is cycled back for reoxidation Alternatively, if the last step is carried out in air 4 NO 2 (g) O 2 (g) 2 H 2 O (l) → 4 HNO 3 (aq) The aqueous HNO 3 obtained can be concentrated by distillation up to about 68




Complete The Following Chemical Reactions I Pbs S H2o2 Aq Ii Mno4 Aq H2o2 Aq Iii Cao S H2o G Iv Alcl3 G H2o L V Ca3n2 S H2o L Classify The Above
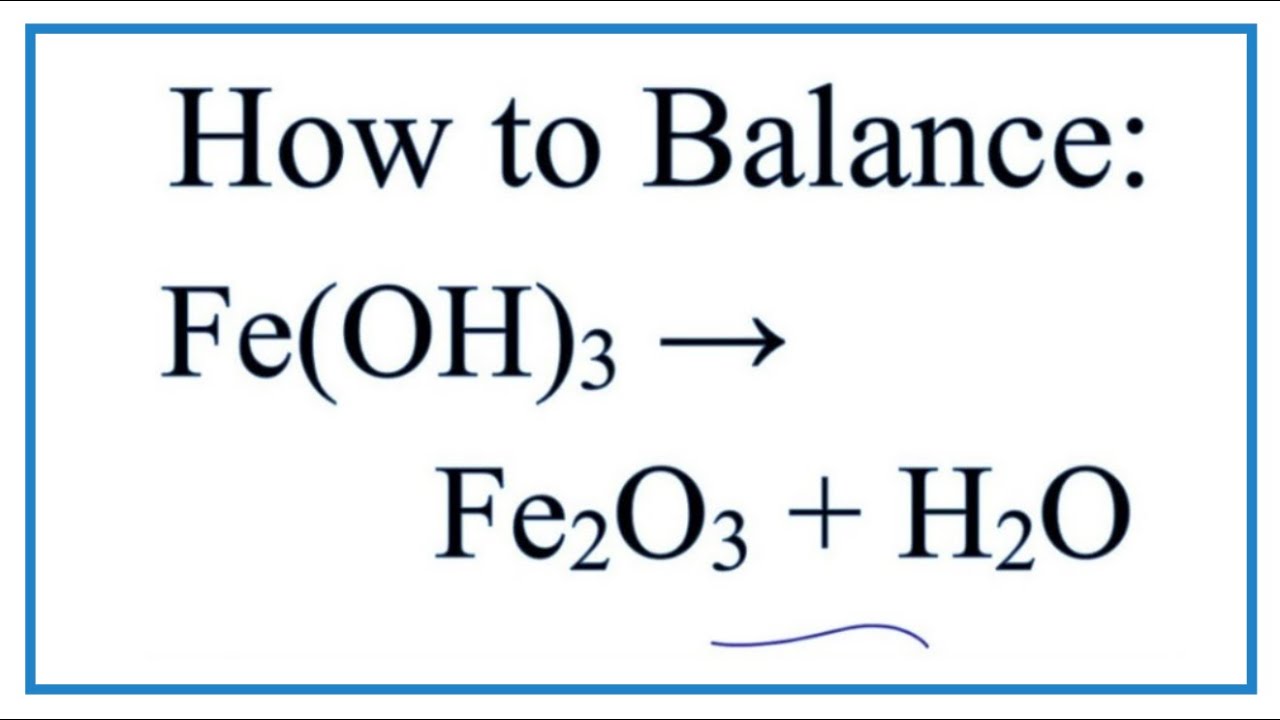



How To Balance Fe Oh 3 And Heat Fe2o3 H2o Decomposition Of Iron Iii Hydroxide Youtube
Al HNO3 Cân bằng phương trình hóa học Hiện tượng Chất rắn màu trắng của nhôm (Al) tan dần trong dung dịch, xuất hiện khí nitơ đioxit (NO2) màu nâu đỏ Lớp 11 Phản ứng oxihoá khửB) HNO3 c) H2O d) OH– e) CH3OH ANS b) HNO3 PAGE 142 14 Identify the strongest base a) CH3O– b) CH3OH c) CN– d) H2O e) NO3– ANS a) CH3O– PAGE 142 15 Given that the Ka for HOCl is 35 x 108, calculate the K value for the reaction of HOCl with OH a) 35 x 106 b) 35 x 108 c) 35 x 1022 d) 29 x 107 e) none of theseChemistry Chemical Reactions Balancing Chemical Equations 1 Answer Adeeb K Co(OH)3 3HNO3 > Co(NO3)3 3H2O Answer link Related questions When balancing equations, which numbers are you allowed to change?



Www Crsd Org Cms Lib5 Pa Centricity Domain 399 Review keys for both Pdf




Ncert Exemplar Class 10 Science Solutions Chapter 1 Download Free Pdf
Kyselina Dusitá HNO 2 Molar Mass of HNO2 Oxidation Numbers of HNO2 Hno2ID Cho phương trình hoá họcAl HNO3 → Al(NO3)3 NO N2O H2O(Biết tỉ lệ thể tích N2O NO = 1 3) Sau khi cân bằng phương trình hoá học trên với hệ số các chất là những số nguyên có ước chung lớn nhất bằng 1 thì hệ số của HNO3 là A 66Find 3(3Pyridyl)Lalanine and related products for scientific research at MilliporeSigma




Dalton




How To Balance Al Oh 3 Hcl Alcl3 H2o Aluminum Hydroxide Hydrochloric Acid Youtube
Find 3,5 DICHLORO2HYDROXYBENZENESULFONIC ACID and related products for scientific research at MilliporeSigmaTrộn 3 dung dịch \(H_2SO_4\) 0,1M ;In this video we'll balance the equation HNO3Ca(OH)2 = Ca(NO3)2H2O and provide the correct coefficients for each compound To balance HNO3Ca(OH)2 = Ca(




R Eenadu Pratibha




Balance Cr Oh 3 H2o2 Cro4 2 H2o Brainly In
HNO 3 Al(OH) 3 = Al(NO 3) 3 H 2 O double replacement HNO 3 Mg(OH) 2 = H 2 O Mg(NO 3) 2 double replacement HNO 3 NaOH = NaNO 3 H 2 O double replacement Formula in Hill system is HNO3 Computing molar mass (molar weight) To calculate molar mass of a chemical compound enter its formula and click 'Compute' In chemical formula you¬¼, ya que al ser un ácido pH 7 y pOH 7!HNO 3 3H 3e→ NO ↑ 2 H 2O 3 H 2O Co 2 NO 3 H e13 REAÇÕES REDOX PARA COMPOSTOS ORGÂNICOS OH H O H O R CDIMINUIÇÃO DO CONTEÚDO EM OXIGÊNIO REAÇÕES REDOX PARA COMPOSTOS ORGÂNICOS Do mesmo modo, a conversão de aldeído em álcool, representa uma redução




Booklet 5 Ch 14 15 16 Covalent Bond Amine




How To Balance H3po4 Mg Oh 2 Mg3 Po4 2 H2o Youtube
Info AL(OH)3 might be an improperly capitalized Al(OH)3 Info AL(NO3)3 might be an improperly capitalized Al(NO3)3, Al(No3)3 Instrucciones y ejemplos siguientes pueden ayudar a resolver este problema Siempre puedes pedir ayuda en el foroC 8 H 18 O 2;0,75 mol H 2O 19 Considera la seguente reazione bilanciata 2Al




Complete The Following Chemical Reactions Classify The Below Into A Hydrolysis B Redox And C Hydration Reactions I Pbs S H2o2 Aq Ii Mno4 Aq H2o2 Aq Iii Cao S H2o




Type Of Reaction For Al Oh 3 Al2o3 H2o At High Temps Youtube
0 al 2 o 3 0 hno 3 h 2 o → 0 aloh 2 (no 3) 2 h 2 o Upozornění Některé sloučeniny nehrají při reakci žádnou roli a mají 0 koeficientů Ujistěte se, že jste rovnici zadali správněVyvažte reakci Ti(OH)3 HNO2 = Ti(NO2)3 H2O pomocí tohoto balanceru chemických rovnic! How would you balance Co(OH)3 HNO3>Co(NO3)3 H2O?



6 5 Classifying Chemical Reactions Redox Problems Chemistry Libretexts




Pdf Metal Promoted Extraction Deprotonation Of Bidentate Organophosphoric Reagents Recovery Of Uranium Thorium And Lanthanides
Question Al(OH)_3 3 HNO_3 Rightarrow Al(NO_3)_3 3 H_2O What Mass Of HNO_3 Is Needed To React With 2 Moles Of Al (OH)_3?Al(oh) 3 3 hno 3 → al(no 3) 3 3 h 2 o Reaction Information Gibbsite Nitric Acid = Aluminum Nitrate WaterHòa tan 4,59 gam Al bằng dung dịch HNO 3 dư thu được hỗn hợp khí NO và N 2 O có tỉ khối hơi đối với hiđro bằng 16,75 (ngoài ra không có sản phẩm khử nào khác) Thể tích (đktc) NO và N 2 O thu được lần lượt là




How To Balance Mg Oh 2 Hno3 Mg No3 2 H2o Magnesium Hydroxide Nitric Acid Youtube



Pdf Microwave Assisted Hydrothermal Synthesis Of Bi6 No3 2o7 Oh 2 And Its Photocatalytic Properties
2HNO Ba(OH) Ba(NO ) 2H O 3 2 3 2 2 o 3 3 1000 mL 1356 0'58 g HNO x 19'662 g HNO 25 mL x o ½ ¾ o ¿ Por la estequiometria de la reacción sabemos que 23 2 3 171'3 g Ba(OH) 2 63 g HNO x 26'73 g Ba(OH) H O OH 3 ªº¬¼ !1) c3h4>c3h5br>c3h6o>c3h8o>c3h6>c3h8 2) c4h6>c4h8o>c4h10o>c4h8>c4h8br2>c4h6 3) c3h4>c3h3na>c5h8>c5h9br>c5h8o>c5h10oAnswer to A chemical reaction is given 2 HNO_{3} (aq) Ba(OH)_{2} (aq) \rightarrow 2 H_{2}O (l) Ba(NO_{3})_{2} (aq) A 01 L sample of an




Cambridge International As A Level Chemistry Student Book Answers Ionic Bonding Chemical Bond



Thesis Library Caltech Edu 19 5 Akmollnerthesis07ch3 Pdf
CH3COOH H2O CH3COO H3O 5 3 3 3 a 1,8·10 CH COOH CH COO H O K C0(M) 1 Ceq (M) 1 x x x 2) Tal y como se ha comentado anteriormente, cuando el ácido proporcione una concentración de H3O > 106 M, la contribución del agua a la concentración de H3O (y OH) del medio será despreciable,7 Al 24 HNO 3 → 7 Al(NO 3) 3 3 NH 2 9 H 2 O Reaction Information Aluminij Dušična Kiselina = Aluminum Nitrate Aminyl Voda Reaktanti Aluminij Al Molar Mass of Al Dušična Kiselina HNO 3 Molar Mass of HNO3 Oxidation Numbers of HNO3 Nitratna Kiselina Proizvodi Aluminum Nitrate2 6 NaNO 3 q) Te 4 HNO 3 2→ TeO 2 H 2O 4NO 2 9 Scrivi e bilancia l'equazione della reazione tra sol (OH) 3(s) → Al 2O 3(s) 3H 2O (g) Quante moli di ossido di alluminio e di acqua si ottengono riscaldando 0,50 mol di Al(OH) 3?




3 Chem Web




How To Balance Cu Hno3 Cu No3 2 No2 H2o Concentrated Hno3 Youtube
In the reaction, HNO_{3}(aq)H_{2}O(l)=H_{3}O^{}NO_{3}^{} the water and hydronium ion are HNO_3 H_2O to HNO_3(aq) to H^ NO^(3) In English nitric acid and water form a solution, it then solvates into its ions in the solution since HNO_3 is solubleHòa tan hoàn toàn m gam Al bằng dung dịch HNO 3 loãng, thu được 5,376 lít (đktc) hỗn hợp khí X gồm N 2, N 2 O và dung dịch chứa 8m gam muối Tỉ khối của X so với H 2 bằng 18 Giá trị của m là A 17,28 B 21,60 C 19,44 D 8,90 Xem đáp án câu 1




6 3 Classifying Chemical Reactions Precipitation Problems Chemistry Libretexts



5 1 Writing And Balancing Chemical Equations Problems Chemistry Libretexts
A H aq HNO 3 aq 2OH aq 2 H 2 O l NO 3 aq B HNO 3 aq NaOH aq NaNO 3 aq H 2 O l C from CH 223 at Portland Community CollegeCâu hỏi Cho phương trình phản ứng Al HNO 3 → Al(NO 3) 3 N 2 O N 2 H 2 O Nếu tỉ lệ số mol N 2 O và N 2 là 2 3 thì sau cân bằng ta có tỉ lệ mol Al N 2 O N 2 làHNO 3 Al(OH) 3 = Al(NO 3) 3 H 2 O doble desplazamiento HNO 3 Mg(OH) 2 = H 2 O Mg(NO 3) 2 doble desplazamiento HNO 3 NaOH = NaNO 3 H 2 O doble desplazamiento Formula en el sistema Hill es HNO3 Calculando la masa molar (peso molecular) Para calcular la masa molar de un compuesto químico introduzca su formula y haga click en




How To Balance Naoh Al Al Oh 3 Na Sodium Hydroxide And Aluminum Youtube



How To Balance Hno3 Al Oh 3 Al No3 3 H2o Youtube
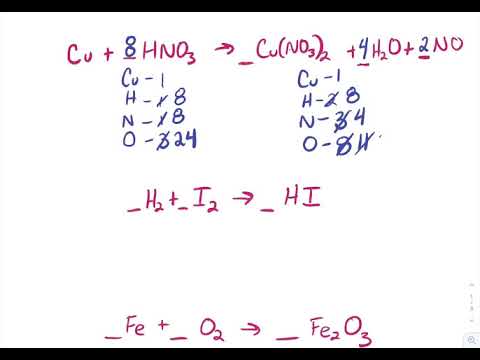



5 1 Writing And Balancing Chemical Equations Problems Chemistry Libretexts




How To Balance Hno3 Ca Oh 2 Ca No3 2 H2o Nitric Acid And Calcium Hydroxide Youtube



1




5 Pages 51 100 Flip Pdf Download Fliphtml5




Chapter 15 Wh Freeman




Chemistry Workbook Manualzz




5 Pages 51 100 Flip Pdf Download Fliphtml5



Mmstcchemistry Weebly Com Uploads 2 4 1 2 Answers Chapter 4 Pdf



Http Ion Chem Usu Edu Scheiner Lundellchemistry Practiceproblems Ch11 Practiceproblems Key Pdf




To Balance Al Oh 3 Hno3 Al No3 3 H2o Nomber Of Molecules Hno3 Required Vwill Be Brainly In



Http Chemistry Oregonstate Edu Courses Ch224 6 Ch226 07 Chapter 19 Pdf




View Sample Chapter Smithers Rapra



Http Ion Chem Usu Edu Scheiner Lundellchemistry Practiceproblems Ch11 Practiceproblems Key Pdf




Answer Key




To Balance Al Oh 3 Hno3 Al No3 3 H2o Nomber Of Molecules Hno3 Required Vwill Be Brainly In




Pdf Direct Measurements Of The Convective Recycling Of The Upper Troposphere




How To Balance H2so4 Al Oh 3 Al2 So4 3 H2o Youtube
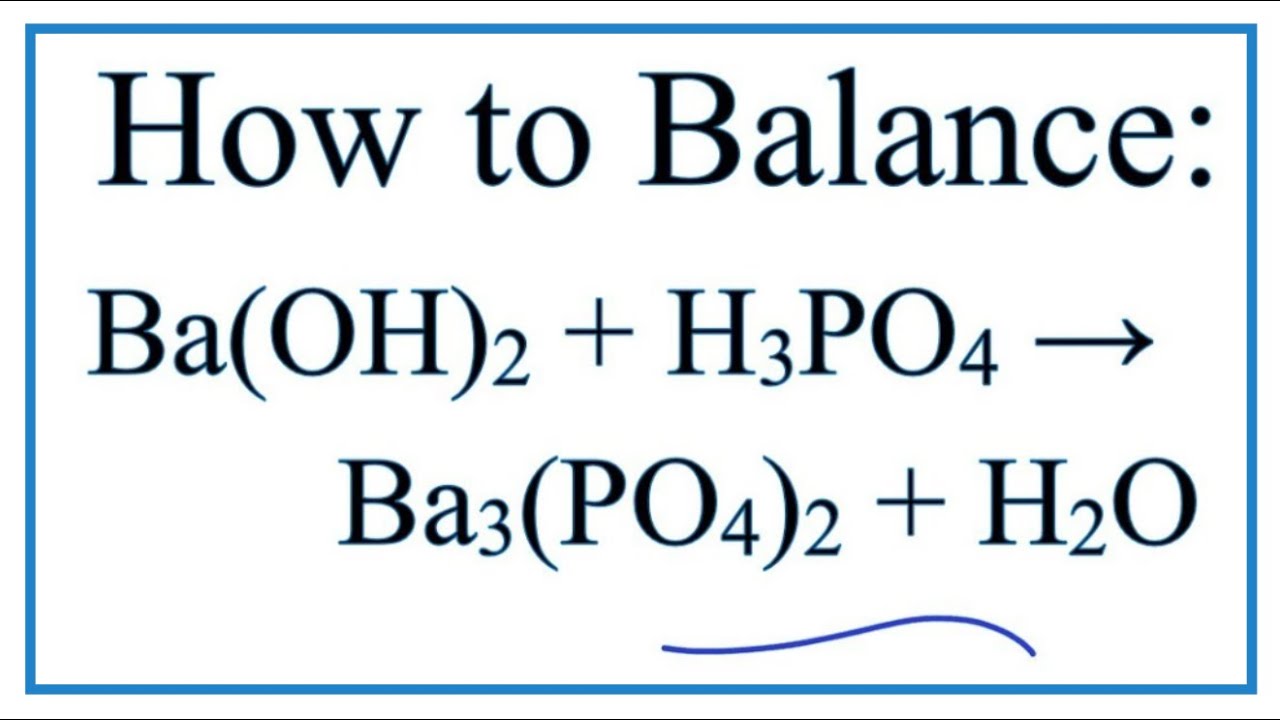



How To Balance Ba Oh 2 H3po4 Ba3 Po4 2 H2o Barium Hydroxide Phosphoric Acid Youtube




Balance Al H2o Al Oh 3 H2 Aluminum And Water Youtube




Consider The Following Reaction 3 No2 G Clutch Prep



Q Tbn And9gcq40dldljfjg mlrfersb61lndvmozytqlxfrtsownifhwana Usqp Cau




New Way Chem Book 4 Public Exam Questions Solution




Write The Balanced Chemical Equations For The Following Reactions A Calcium Hydroxide Carbon Dioxide Calcium Carbonate Water B Zinc Silver Nitrate Zinc Nitrate Silver C Aluminium Copper Chloride




11 Key




Chapter 3 Mass Relationships In Chemical Reactions




11 Key
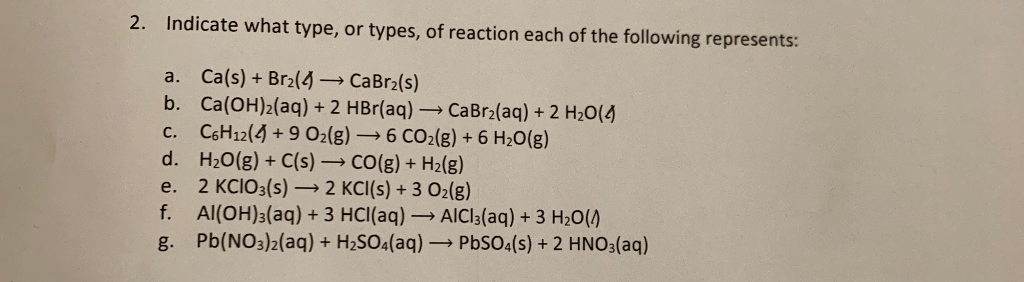



2 Indicate What Type Or Types Of Reaction Each Of Chegg Com




Lanthanum Hydroxide Wikipedia




6 5 Classifying Chemical Reactions Redox Problems Chemistry Libretexts




Balance A Redox Reaction Basic Solution Youtube




Alumminum Hydroxide Reacts With Sulfuric Acid As Follows 2al Oh 3 H2so4 Al2 So4 6h2o Which Reagent Is The Limiting Reactant When 0 500 Mol Al Oh 3 And 0 500 Mol H2so4 Are Allowed To React How Ma Study Com
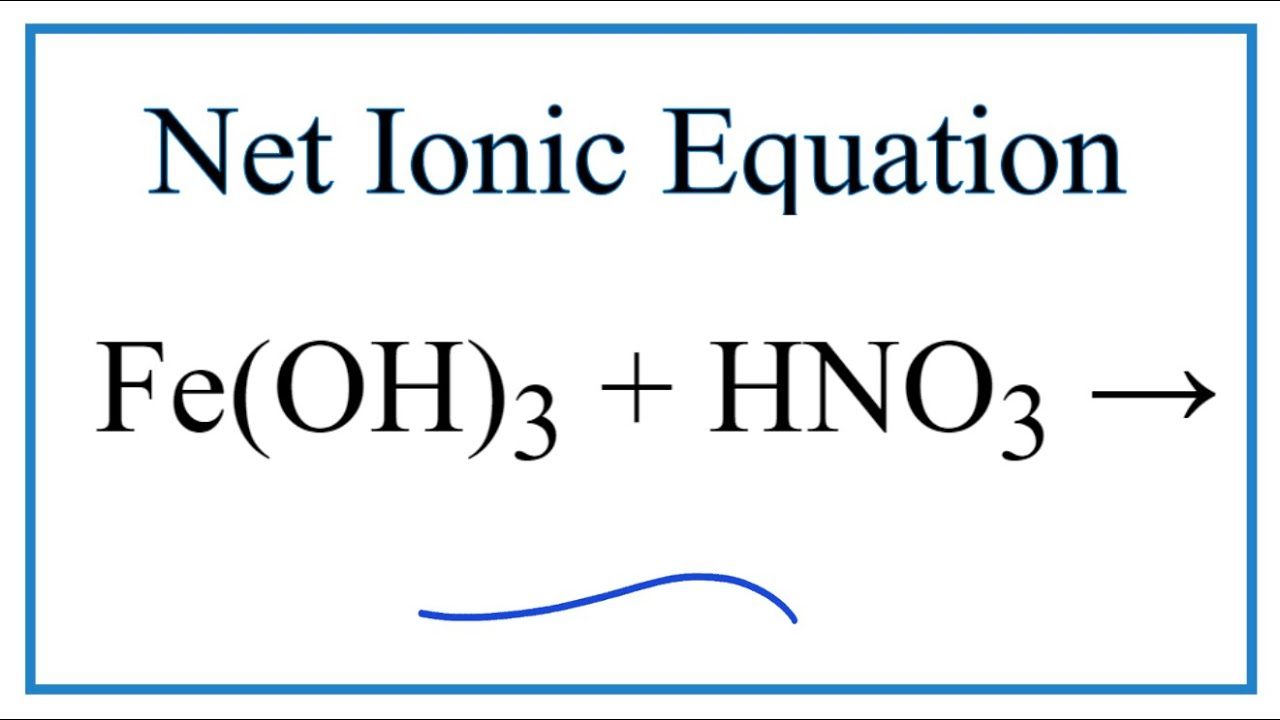



How To Write The Net Ionic Equation For Fe Oh 3 Hno3 Fe No3 3 H2o Youtube




P Block12 457 Pdf Chlorine Nitric Acid



5 1 Writing And Balancing Chemical Equations Problems Chemistry Libretexts




Extended Abstract Ams Supported Meetings




Pdf Heterogeneous Conversion Of No2 And No On Hno3 Treated Soot Surfaces Atmospheric Implications



Www Scasd Org Cms Lib5 Pa Centricity Domain 18 11 review sheet Pdf



2



Molecules Compounds And Chemical Equations Chapter 3




How To Balance Al Oh 3 Al2o3 H2o At High Temperatures Youtube



1
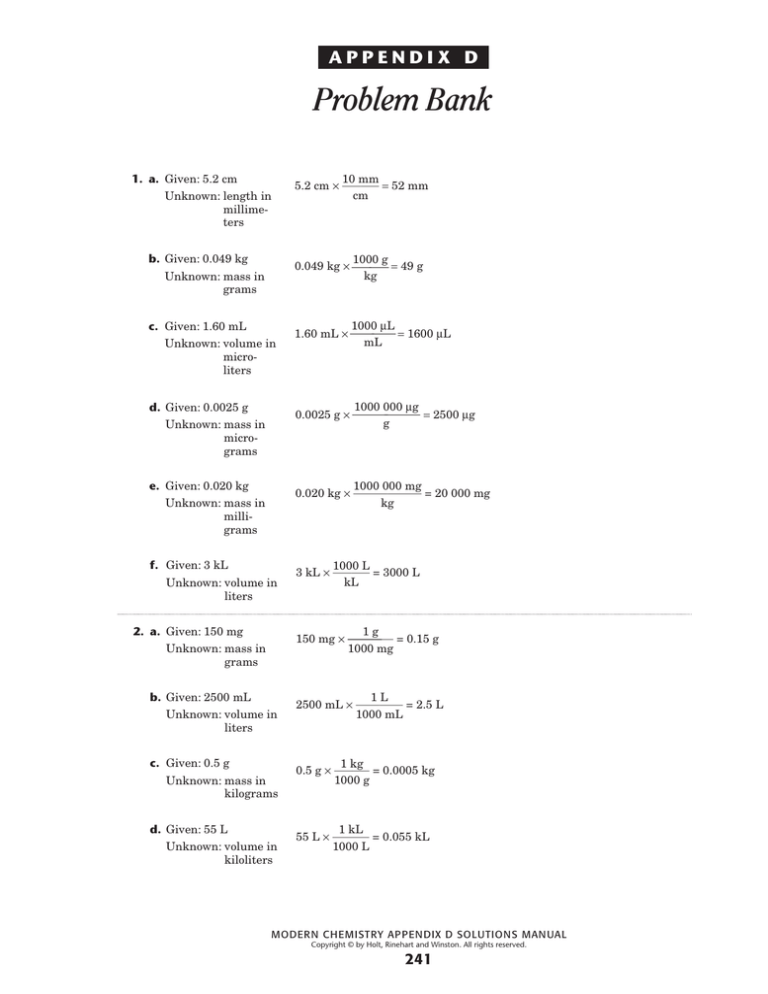



Holt Modern Chemistry Appendix D Problem Bank Solutions




Balance The Following Redox Reactions Occu Clutch Prep




Chemistry Lesson Plans 08 Stoichiometry The Fansler S




Pdf Hydrogen Bonded Complexes Between Nitrogen Dioxide Nitric Acid Nitrous Acid And Water With Sih3oh And Si Oh 4electronic Supplementary Information Esi Available Structural Data For Si Oh 4 Sih3oh Hno3 Hono No2 N2o4 H2o Si Oh 4



Http Www Profpaz Com Files Chem52 Hw 17ans Pdf
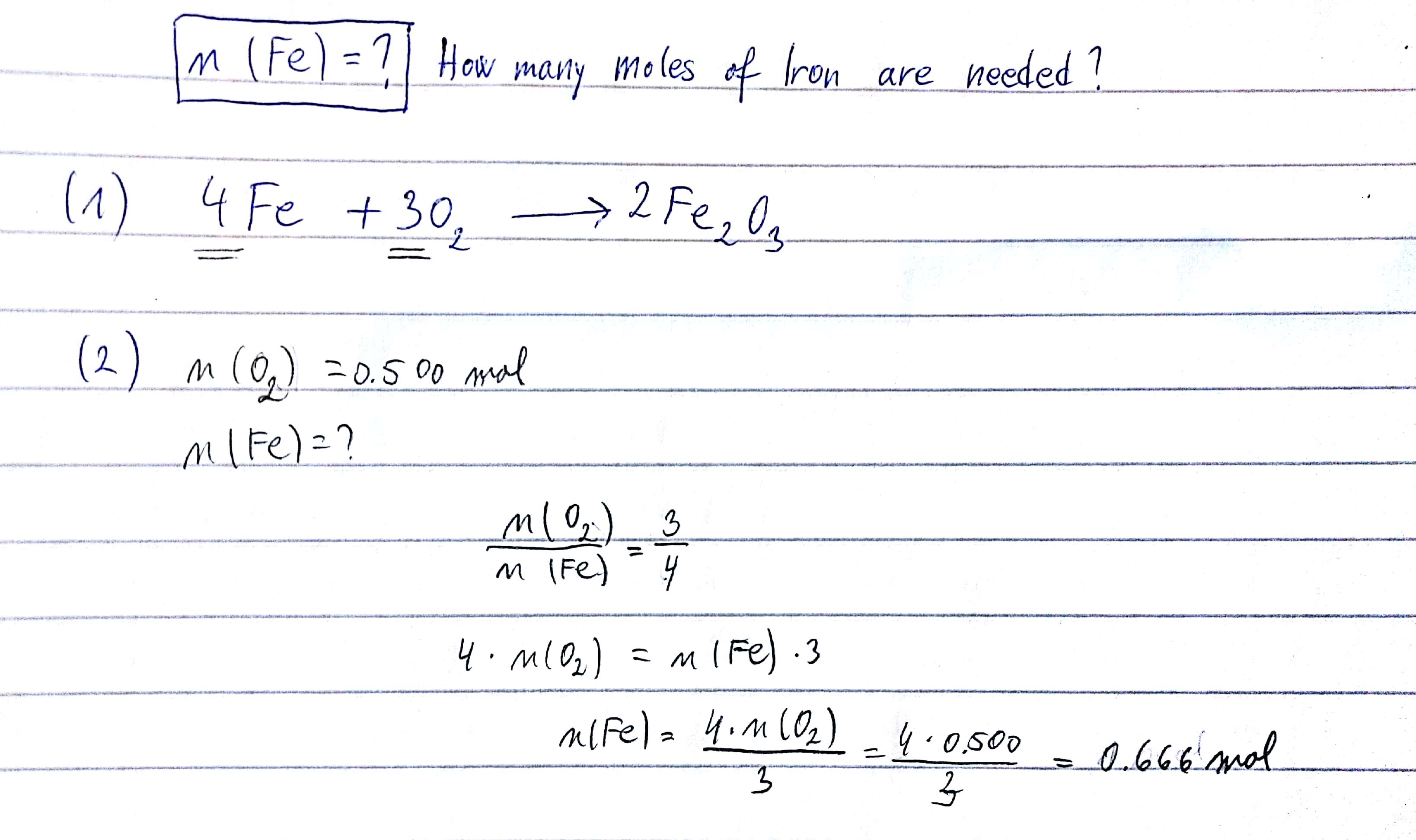



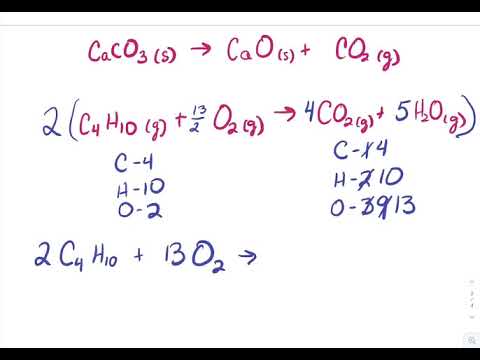
How Do You Balance The Equation 4 Fe S 3 O2 G 2 Fe2o3 S Socratic




Oxidation Reduction Equations Boundless Chemistry




Copper Ii Carbonate Wikipedia



Www Elcamino Edu Faculty Abalakin Textbook problems solutions Chapter 5 Pdf




How To Balance Al Oh 3 Hcl Alcl3 H2o Aluminum Hydroxide Hydrochloric Acid Youtube




528 68 Grams Of Nh So J Balance The Following Chegg Com
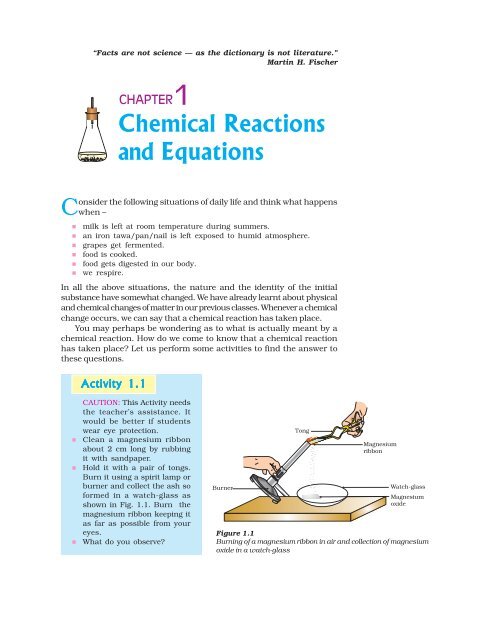



Jesc101




How To Balance Hno3 Zn Zn No3 2 H2 Note Dilute Hno3 Is Used Youtube
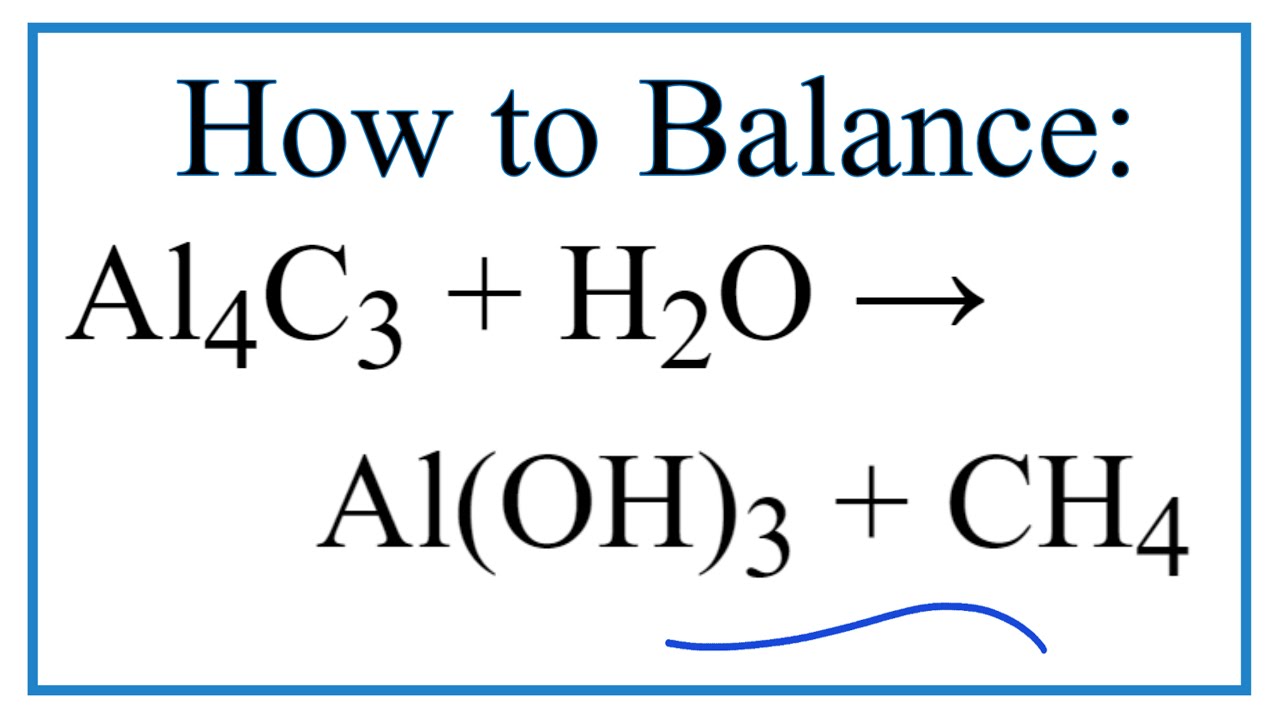



How To Balance Al4c3 H2o Al Oh 3 Ch4 Aluminum Carbide Water Youtube
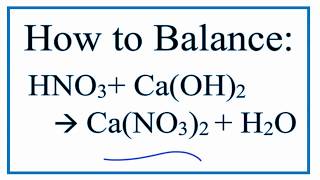



How To Balance Hno3 Ca Oh 2 Ca No3 2 H2o Nitric Acid And Calcium Hydroxide Youtube




Hono Yield In The Reaction Of No 2 With Degussa Lamp Black 101 Treated Download Scientific Diagram




11 Key




Type Of Reaction For Al Oh 3 Al2o3 H2o At High Temps Youtube




Pdf Heterogeneous Conversion Of No2 And No On Hno3 Treated Soot Surfaces Atmospheric Implications



Www Geneseocsd Org Site Handlers Filedownload Ashx Moduleinstanceid 196 Dataid 1019 Filename Practice test redox Pdf




Experimental Set Up Download Scientific Diagram




How To Balance Hno3 Al Oh 3 Al No3 3 H2o Youtube




5 1 Writing And Balancing Chemical Equations Problems Chemistry Libretexts




Equation For Al Oh 3 H2o Aluminum Hydroxide Water Youtube




Chemistry Methyl Group Ether




Alcohols Ethers And Phenols Sakshieducation Com




Chemistry Of Inorganic Nitrogen Compounds Outline




Experimental Set Up Download Scientific Diagram



Http Brearleyhigh Kenilworthschools Com Userfiles Servers Server 7985 File Mr novak 27s chemistry Ch 8 study guide answer key Study Gd Ak Pdf



0 件のコメント:
コメントを投稿